2021 AEA Seed Grant Final Report: Dr. Soon-Tae Lee
May 27, 2022

It was May 14, 2021 when we introduced Dr. Soon-Tae Lee. Click here for last year’s post where we learned about him, his study plans and how his project would help patients and families affected by AE.
In the 2021 AEA Community Seed Grant Final Report, Dr. Lee explains the outcomes of his project, Development of optimal clinical approaches in seronegative autoimmune encephalitis.
1. List the specific aims of your project and explain how they were met.
Study aims: Seronegative autoimmune encephalitis (AE) is AE without any identifiable pathogenic antibody. Although it is a major subtype of AE, many unmet clinical needs exist in terms of clinical characteristics, treatments, and prognosis. The current clinical study was to investigate the following subjects. (1) Clinical spectrum of seronegative AE, (2) Efficacy of immunotherapeutic drugs, (3) Optimal duration of immunotherapy, (4) Optimal interval of MRI, (5) Development of prognosis prediction score.
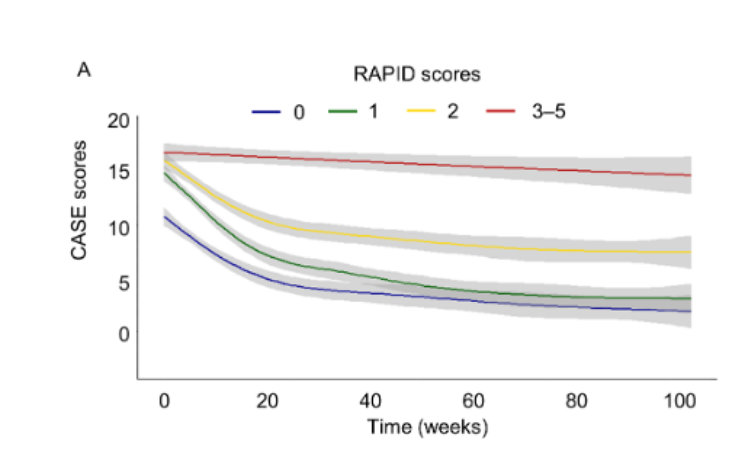
Study results: Seronegative AE was subcategorized into antibody-negative probable AE (ANPRA), autoimmune limbic encephalitis (LE), and acute disseminated encephalomyelitis (ADEM). The poor 2-year outcome was defined by modified Rankin scale [mRS] scores 3‒6, and the 2-year serial data of Clinical Assessment Scales in Autoimmune Encephalitis (CASE) score was used for longitudinal data analyses. A total of 147 patients were included. The frequency of achieving a good 2-year outcome (mRS 0‒2) was 56.5%. The ANPRA subtype exhibited the poorest outcomes, although the baseline severity was similar among the subtypes. The RAPID score consists of five early utilizable clinical factors, refractory status epilepticus, age of onset ≥ 60 years, probable AE (ANPRA subtype), infratentorial involvement, and delay of immunotherapy ≥ 1 month, which was associated with poorer 2-year outcomes. Any immunotherapy was associated with clinical improvement in the patients with low risk for poor 2-year outcomes (RAPID scores 0–1), and the combination immunotherapy of steroid, immunoglobulin, rituximab, and tocilizumab was associated with better outcomes in the patients with high risk for poor 2-year outcomes (RAPID scores 2-5). In patients with persistent disease at 6 months, continuing immunotherapy was associated with more improvement, while the effect of continuing immunotherapy for more than 12 months was unclear. In the longitudinal analysis of MRI, the development of cerebellar atrophy indicated poor outcomes, while the absence of diffuse cerebral atrophy or medial temporal atrophy indicated the possibility of a good outcome
* Access the publication, Seronegative autoimmune encephalitis: clinical characteristics and factors associated with outcomes. Brain, awac166, https://doi.org/10.1093/brain/awac166.
2. Describe the proposed impact/relevance of the project and the outcome.
The proposed impact/relevance of the project: Although the number of patients is large and clinical experiences are accumulating, very little has been investigated about the seronegative AE. Accordingly, not only the patients/families but also the treating physicians confront diagnostic uncertainty, difficulty in deciding on appropriate immunotherapy, and inability to predict the prognosis. By providing the answers to the study aims, physicians, patients, and families could predict the prognosis of each patient, decide which immunotherapy is better at each step, and monitor the patients according to an evidence-based protocol. In this way, seronegative AE patients will be able to have more predictable care, as like those with seropositive AE.
The outcome of the study: This study provided information about the clinical characteristics and courses, the effect of immunotherapy and its duration, and prognostic factors in seronegative AE. Despite the complexness of the disease, now we can say that seronegative AE is conceptualized. The disease is now more predictable and physicians can make more evidence-based decisions and prognostications.
3. Explain how the results of your project have direct implications for patients with AE.
This study comprehensively describes the features, courses, and prognosis of seronegative AE based on a large cohort defined by established diagnostic criteria. The current study provides the following important information about the diagnosis and treatment of seronegative AE, all of which have direct implications for the patients. First, the frequency of good 2-year outcomes was 56.5%, and the ANPRA subtype exhibited the poorest outcomes. Second, RAPID scores consisting of five early utilizable clinical factors (RSE, Age of onset ≥ 60 years, Ab-negative Probable AE (ANPRA) subtype, Infra-tentorial involvement in brain MRI, and Delay of immunotherapy for ≥ 1 month) were associated with poorer 2-year clinical outcomes. Third, the immunotherapy using steroids, IVIG, rituximab, or tocilizumab was effective in the disease, and the combined immunotherapy was feasible, especially in patients with a high risk for poor outcomes at baseline (RAPID scores of 2–5). Fourth, further immunotherapy might be effective for improving outcomes in cases of persistent disease at 6 months, while the effect of further immunotherapy after 12 months was unclear. Fifth, the development of cerebellar atrophy indicated poor outcomes, while the absence of DCA or mTA indicated a possibility of recovery.
4. How did the AEA Community Seed Grant contribute to your ability to complete this project?
The grant supported the study initiation, data acquisition, and publication, along with a principal investigator’s own grant. The study is to be published in a journal with this acknowledgment.
Thank You, Thank You, Thank You!
Thank you, Dr. Soon-Tae Lee for your research and your commitment to improving the lives of patients and families impacted by AE.
Thank you to the entire AE Alliance Community for contributing during the 2020 Research Network Month which assisted in funding this seed grant project.
Thank you for your contributions this year to fund future research!
Together, we are changing the course of AE.